Physical Properties of Acetic Acid
Molecular Formula- CH3COOH( C2H4O2)
Molecular Weight- 60.05 g/mol
Boiling Point- 117.9 °C
Melting Point- 16.635 °C
Solubility- Miscible with Water
Relative vapour density (air = 1)- 2.1
Vapour Pressure- 15.7 mmHg at 25 °C
Stability- Stable under normal laboratory storage conditions
Viscosity- 1.056 mPa.s at 25 °C
Refractive Index- 1.3720 @ °C/D
Surface Tension- 27.10 mN/m at 25 °C
pH- 1.0 molar = 2.4; 0.1 molar = 2.9; 0.01 molar = 3.4
Structure
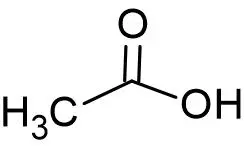 Overview
Overview
- Acetic acid, also known as ethanoic acid, is a byproduct of fermentation.
- Acetic acid is characterized by its colourless liquid nature and possesses a pungent odour, along with a distinctive burning taste.
- Acetic acid constitutes approximately 4-6% of the solution in vinegar.
- More concentrated forms are used in laboratory settings.
- Pure acetic acid without water is called glacial acetic acid.
- Dilute solutions like vinegar are generally safe for skin contact.
- Concentrated solutions can cause skin burns.
- Glacial acetic acid can induce skin burns, cause permanent eye damage, and corrode metal.
Natural Sources of Acetic Acid
- Acetates, which are salts of acetic acid, are frequently found in animal and plant tissues and are generated during the metabolism of food.
- Most tissues readily metabolize acetate, leading to the production of ketones as intermediates.
- The body utilizes acetate as a fundamental building block for synthesising phospholipids, neutral lipids, steroids, sterols, and saturated, and unsaturated fatty acids.
- This utilization occurs across a range of human and animal tissue preparations.
Also Read: Unlocking the Potential of Artificial DNA “Breakthrough in Genetic Transcription” – Know More (kmore.info)
Effects of Acetic Acid exposure
- Low concentrations of acetic acid found in foods like vinegar are generally harmless.
- Higher concentrations, common in laboratory or industrial settings, can be a strong irritant to eyes, skin, and mucous membranes.
- Prolonged skin contact with concentrated acetic acid may lead to tissue destruction.
- Inhalation of high concentrations of acetic acid vapours can irritate the eyes, nose, and throat.
- Individuals with high occupational exposure may develop conjunctivitis, bronchitis, pharyngitis, and erosion of exposed teeth (incisors and canines).
Uses of Acetic Acid
- Acetic acid is key in producing acetic anhydride, cellulose acetate, vinyl acetate monomer, and other chemical compounds.
- It is utilized in the manufacturing of plastics, dyes, insecticides, photographic chemicals, and rubber.
- Additionally, acetic acid is involved in the production of vitamins, antibiotics, hormones, and various organic chemicals.
- It serves as a food additive, specifically as an acidulant.
- Acetic acid finds application in diverse textile printing processes.
Food Preparation:
– Vinegar serves as a common food ingredient, utilized in pickling liquids, vinaigrettes, marinades, and salad dressings.
– It aids in controlling Salmonella contamination in meat and poultry products during food preparation.
Cleaning:
– Vinegar finds versatile use throughout the home, acting as a window cleaner, descaling agent for coffee makers, and dish cleaner.
– It serves as a rinsing agent for dishwashers and effectively cleans bathroom tile, grout, and food-related tools.
– Vinegar’s residue is generally non-harmful, requiring less rinsing during cleaning.
Gardening:
– In concentrations of 10 to 20%, can function as a weed killer in gardens and lawns.
– As a herbicide, it eliminates emerged weeds without affecting their roots, allowing for potential regrowth.

Thank you for your articles. They are very helpful to me. Can you help me with something?
thanks…..yeah let me know what can i do
Thank you for your articles. They are very helpful to me. Can you help me with something?
Great beat ! I would like to apprentice while you amend your web site, how could i subscribe for a blog site? The account helped me a acceptable deal. I had been a little bit acquainted of this your broadcast provided bright clear concept
added you in my subscriber list
Thank you for your articles. They are very helpful to me. Can you help me with something?
what happened i will try to help if possible
Great beat ! I would like to apprentice while you amend your web site, how could i subscribe for a blog site? The account helped me a acceptable deal. I had been a little bit acquainted of this your broadcast provided bright clear concept
i have added you in my subscriber list
Thank you for providing me with these article examples. May I ask you a question?
😊yeah what’s your question
Great content! Super high-quality! Keep it up!
😊 thanks do share
I want to thank you for your assistance and this post. It’s been great.
Thanks